Market Opportunity
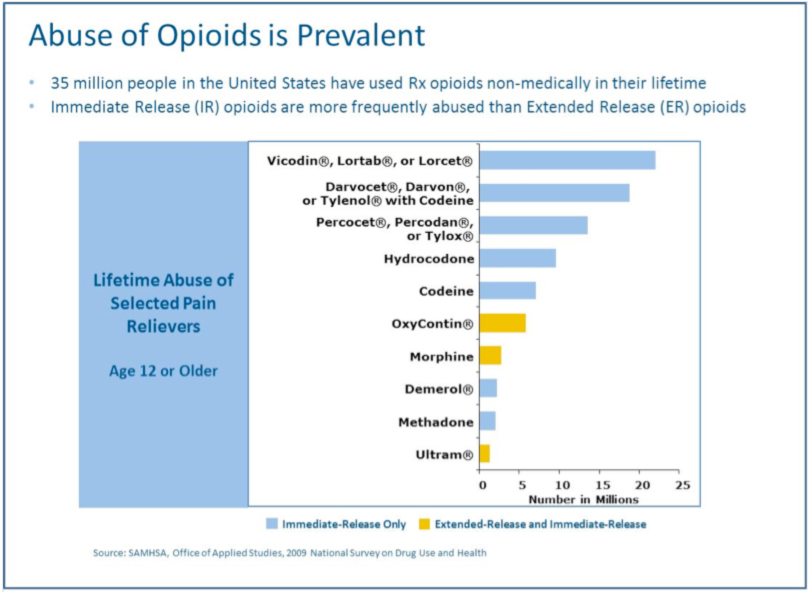
Opioid Analgesics
In a 2011 survey of 400 opioid prescribing physicians, 39% of physicians indicated that they were highly concerned with the diversion of their opioid prescriptions for non-medical purposes and 42% were highly concerned about opioid abuse by their patients. Less than 17% of these same physicians indicated they were confident they could adequately identify patients who are diverting or misusing their opioid prescriptions. Further, 77% and 66% of the physicians indicated that abuse of their opioid prescription by injection and snorting, respectively, would likely lead to serious adverse health consequences for the abuser as compared to only 38% for abuse by oral administration.
Several independent organizations have estimated the potential cost impact of prescription opioid abuse to insurers. An analysis of health and pharmacy insurance claims between 1998 and 2002 for almost two million Americans conducted by Analysis Group, Inc. and others indicated that enrollees with a diagnosis of opioid abuse had average claims of approximately $14,000 per year higher than an age-gender matched non-opioid abuse sample. A 2007 report by the Coalition Against Insurance Fraud, after adjusting for inflation, estimated this excess cost per patient at more than $16,000 for 2007. By applying the U.S. government’s estimated 4.4 million annual opioid abusers, this organization concluded that abuse of IR and ER opioid products could cost health insurers up to $72.5 billion a year.
More…
Nasal Decongestants
Pseudoephedrine (PSE) is a widely-used nasal decongestant available in many non-prescription and prescription cold, sinus and allergy products. PSE products can be misused for the clandestine production of methamphetamine, a highly addictive, illicit drug abused by an estimated 1.1 million Americans annually.
In 2006, regulations relating to over-the counter sale of PSE products were amended with the enactment of the Federal Combat Methamphetamine Epidemic Act (CMEA). The CMEA was enacted in response to an alarming increase in and widespread conversion of PSE containing products into methamphetamine. Among other things, the CMEA requires retail stores to maintain their inventory of PSE containing products in a secured location and restricts the amount of PSE products a store can sell to an individual customer. NEXAFED®, Acura’s PSE tablet product, uses the company’s IMPEDE® Technology to deter the conversion of PSE into methamphetamine. Acura expects NEXAFED® to compete in the market for cold, sinus and allergy products available to consumers without a prescription. In 2009, AC Nielsen reported approximately $1.0 billion in sales of non-prescription products containing either PSE or phenylephrine as a nasal decongestant, of which approximately 47% contained PSE. In Acura’s 2012 survey of 215 chain and independent pharmacists, 164 indicated they had influence over the pharmacies’ product offerings. Of such pharmacists, 70% indicated they were likely to stock or recommend stocking NEXAFED® in their pharmacies. The 215 surveyed pharmacists also indicated a willingness to recommend NEXAFED® to over 50% of their customers who seek a pharmacist’s advice for a single ingredient nasal decongestant. A separate market study conducted on Acura’s behalf showed that approximately one-third of consumers seek a pharmacist’s recommendation for their nasal decongestant and, as a result of the CMEA, are required to do so to purchase PSE containing products.
Acura is capitalizing on this consumer-pharmacist interaction at the point of sale by soliciting distribution to the pharmacies and engaging pharmacists to recommend NEXAFED® to their customers.
The Company is marketing NEXAFED® pursuant to the FDA’s OTC Monograph regulations.