Study AP-ADF-106
| Product: | Acurox® Tablets |
| Study: | AP-ADF-106 or Study 106 |
| Phase: | Phase I |
| Title: | A Phase I Single-Center, Single-Blind Study in Recreational Opioid Users to Evaluate the Safety, Tolerability, and Pharmacokinetics of Crushed and Intranasally Administered Acurox (oxycodone HCl and niacin) Tablets |
Study Objective
To assess the potential abuse liability of intranasally administered crushed Acurox Tablets, crushed generic oxycodone HCl tablets and pure oxycodone HCl powder.
Design Summary
Study 106 was a two part clinical study in subjects with a history of recreational intranasal opioid use. The primary objective of Part 1 was to determine the maximum tolerated dose of crushed Acurox Tablets that subjects could snort in a single administration. Subjects were administered escalating doses of crushed Acurox Tablets (7.5/30 mg) starting at one half tablet and increasing in half tablet increments on successive days. Part 1 also assessed (over 8 hours after administration) vital signs, subjective ratings of liking and somatic (bodily) discomfort, and objective assessments of the nasal cavity determined by endoscopy and intranasal photography.
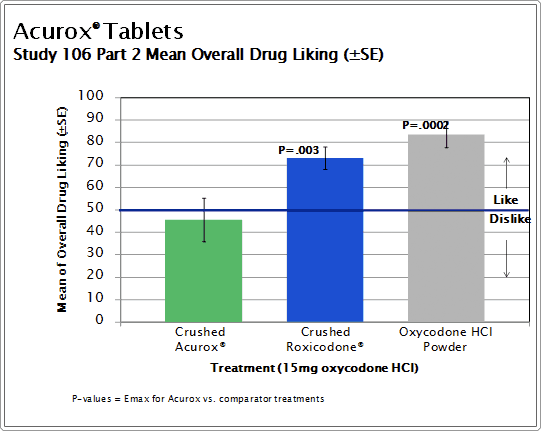
Part 2 assessed the relative abuse liability of intranasally administered crushed Acurox® Tablets (7.5/30 mg), crushed generic oxycodone HCl immediate release tablets and pure oxycodone HCl powder, all with a quantity of oxycodone HCl equivalent to the group median highest tolerated dose of Acurox® Tablets determined in Part 1. Part 2 measurements were made over 12 hours and included vital signs, subjective measures of liking and somatic (bodily) discomfort, objective assessments of the nasal cavity and the pharmacokinetics of oxycodone after intranasal administration.
Results Summary
Part 1 of the study (15 subjects enrolled, 13 subjects completed, 14 subjects analyzed) determined the maximum tolerated intranasal dose of crushed Acurox® Tablets was 15 mg oxycodone HCl/60 mg niacin (i.e. 2 × 7.5/30 mg Acurox® Tablets). The investigator concluded it was not in the subjects’ best interests to continue to higher levels due to the severity of side effects as weighed against the study objectives. Objective and subjective assessments indicated nasal congestion, irritation, discharge, and burning. These effects were temporary and were most pronounced at 30 minutes after dosing with a return to baseline levels by 1 hour. The overall drug effects (Overall Drug Liking, Overall Drug Experience, and Take Drug Again) were measured on a 100 point visual analogue scale (VAS) assessed 8 hours post-dosing. Increasing Acurox® doses were associated with a decrease in liking scores and a decrease in willingness to take the drug again. The Overall Drug Experience was disliked (VAS<50) at every drug dose (0.5, 1, 1.5, and 2 crushed Acurox® Tablets).
In Part 2 of the study (15 subjects enrolled, 12 subjects completed, 10 subjects analyzed), nasal administration of 2 crushed Acurox® Tablets (7.5/30 mg ) resulted in disliking and low positive drug effect on the Overall Drug Experience and Overall Drug Liking scales. In comparison, crushed generic oxycodone HCl tablets and oxycodone HCl powder resulted in high drug liking and positive drug effects with the mean difference being statistically significant at p ≤ 0.0035. Furthermore, subjects were much less willing to take crushed Acurox® Tablets again compared to crushed oxycodone tablets and oxycodone powder (p ≤ 0.0007). Intranasal administration of crushed Acurox® Tablets caused significantly greater negative objective effects for nasal congestion and discharge (p ≤ 0.0223) and negative subjective effects for nasal burning, congestion and need to blow nose (p ≤ 0.0038). The results were not statistically significant for the objective assessment of nasal irritation.
Examination of endoscopy and external photography revealed visible differences between the treatments as only the crushed Acurox® treatment was associated with white foamy substance in the interior and middle turbinate of the nose and visible facial flushing. Oxycodone bioavailability was similar between all three treatments. In both Parts of the study, no serious adverse events were reported. All adverse events were classified as mild or moderate with the most prevalent adverse events being nasal symptoms (congestion, discomfort, discharge), flushing, tearing and euphoria.
The principal investigator’s overall conclusion was that, based on Study 106 results, intranasal administration of crushed Acurox® Tablets has a distinctively lower abuse potential than the same oxycodone HCl dose administered as crushed generic oxycodone HCl tablets or pure oxycodone HCl powder.