Study AP-ADF-111
| Product: | Acurox® Tablets |
| Study: | ADF-111 or Study 111 |
| Phase: | Phase II |
| Title: | A Phase II, Single-Center, Randomized, Double-Blind, Assessment of the Abuse Liability of Acurox (oxycodone HCl and niacin) Tablets in Subjects with a History of Opioid Abuse |
Study Objective
To assess the effects of oxycodone HCl on the effects of niacin (Sequence 1) and the abuse liability and abuse deterrence potential of Acurox Tablets versus oxycodone HCl alone (Sequence 2).
Design Summary
In Study 111, 30 fasted subjects with a history of opioid abuse received a single dose of study drugs every 48 hours for 9 days and were enrolled in two dosing sequences. The first dosing sequence (Sequence 1) included randomized doses of (i) niacin 240mg alone; (ii) a combination of oxycodone HCl 40mg with niacin 240mg (4 times the expected recommended dose of Acurox Tablets 5/30mg); and (iii) placebo tablets. The second dosing sequence (Sequence 2) included randomized doses of (i) a combination of oxycodone HCl 40mg with niacin 240mg (4 times the expected recommended dose of Acurox Tablets 5/30mg) and (ii) oxycodone HCl 40mg alone. On each dosing day, vital sign measures and subjective and behavioral effects were assessed before dosing (baseline) and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, and 12 hours after dosing. Vital signs included measurement of pupil size, blood pressure, heart rate, oral temperature and respiratory rate. For both Sequence 1 and Sequence 2, subjective changes were measured with a two item Drug Rating Questionnaire-Subject (DRQS) and a 40 item short form of the Addiction Research Center Inventory (ARCI). The ARCI was comprised of three scale scores including the Morphine Benzedrine Group scale (MBG) measuring euphoria, the LSD/dysphoria scale measuring somatic/bodily discomfort and dysphoria and the Pentobarbital Chlorpromazine Alcohol Group scale (PCAG) measuring apathetic sedation. For Sequence 2 only, in addition to the DRQS and ARCI, subjects also completed a Street Value Assessment Questionnaire and a Treatment Enjoyment Assessment Questionnaire.
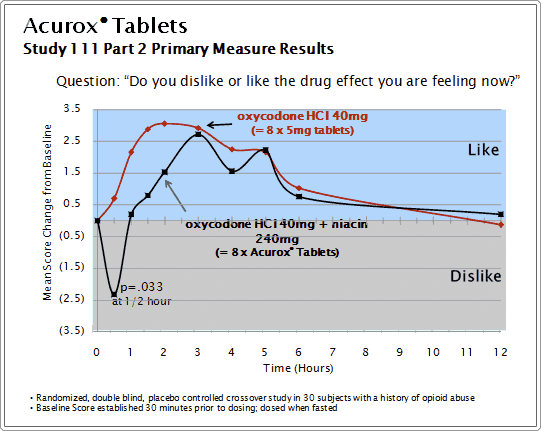
Results Summary
Sequence 1 results demonstrated that response to niacin 240 mg alone compared to placebo causes significant dislike scores (p = .03), and significant LSD/dysphoria scores (p < .001) with these negative niacin induced effects manifesting rapidly, reaching peak at 0.5-1.5 hours and thereafter diminishing. At 0.5 hours after drug administration, oxycodone HCl 40 mg has limited effect on niacin-induced disliking and dysphoric effects. At the one hour observation and afterward, oxycodone may attenuate niacin-induced disliking and dysphoric effects.
Sequence 2 demonstrated that the combination of oxycodone HCl 40mg and niacin 240mg (4 times the expected recommended dose of Acurox Tablets 5/30mg) had the potential to be aversive when compared to oxycodone HCl 40mg alone as shown by statistically significant and clinically meaningful results in the dislike/like scores (p = .033), the Treatment Enjoyment Assessment scores (p = .005) and the LSD/dysphoria scores (p<.001). The dislike/like score at 0.5 hours was designated the primary measure of abuse liability and abuse deterrence potential for Acurox Tablets 5/30mg and the Treatment Enjoyment Assessment scores and LSD/dysphoria scores at 0.5 hours were additional measures of the abuse deterrence potential of Acurox Tablets. Subjective measures not achieving statistical significance included the MBG scores measuring euphoria, the PCAG score measuring apathetic sedation and the Street Value Assessment Questionnaire score, in which subjects indicated they would pay more for oxycodone HCl alone compared to Acurox Tablets (p=.097).
There were no serious adverse events reported. Alterations by niacin compared to placebo on vital signs were minimal and not clinically meaningful. The differences in vital signs between oxycodone HCl/niacin and niacin alone at 4 times the expected recommended dose of Acurox Tablets were minimal and not clinically meaningful.